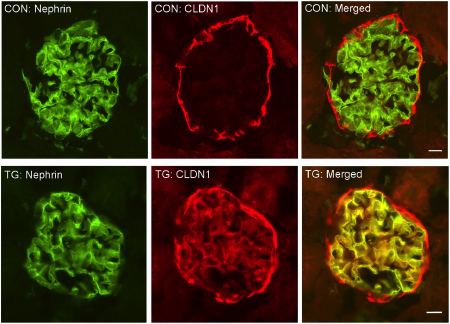
Drs. Yongfeng Gong and Jianghui Hou from the Division of Nephrology have solved a mystery concerning a genetic link between the claudin-1 gene and diabetic kidney disease and proteinuria. Claudin-1 is the major structural and functional component of the eoithelial tight junction, and has been known to be upregulated in podocytes after injury and including in diabetic nephropathy. Gong and Hou created a transgenic mouse with podocyte-specific inducible Claudin-1 expression. They used this novel mouse model to reveal that claudin-1 replaces the cell-cell junction made by the glomerular podocytes known as the slit diaphragm with a new type of cell junction – the tight junction. This cellular architectural change profoundly altered the glomerular permeability to albumin and water. The consequence is uncontrolled proteinuria.
Knowing this novel pathogenic mechanism, it may be possible to design drugs to curb the claudin-1 expression and function in kidney glomeruli. This approach might significantly reduce or prevent the development of proteinuria, which is often the trigger for many more deleterious changes in the kidney, leading to chronic kidney disease and renal failure.