 Angiopoietin-1 (Ang-1), a vascular growth factor secreted by kidney proximal tubular cells, pericytes and podocytes, is essential for regulating blood vessel development and repair after injury. Ang-1 is also a promising therapeutic agent to target renal fibrosis, according to a study by WU researchers recently published in PLoSOne,
Angiopoietin-1 (Ang-1), a vascular growth factor secreted by kidney proximal tubular cells, pericytes and podocytes, is essential for regulating blood vessel development and repair after injury. Ang-1 is also a promising therapeutic agent to target renal fibrosis, according to a study by WU researchers recently published in PLoSOne,
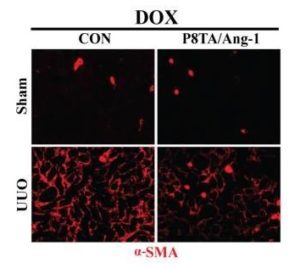
As a result of injury to the renal peritubular capillary network, capillary rarefaction compromises normal kidney function and is pivotal to the development of interstitial fibrosis and CKD progression. Previous studies have shown that certain growth factors or cytokines can protect endothelial cells against renal fibrosis.
The WU research team headed by Ying Maggie Chen, MD, PhD, Assistant Professor of Medicine, Division of Nephrology, generated doxycycline-inducible Ang-1 transgenic mice to examine the therapeutic role of Ang-1 in the renal fibrogenic process and the impact on the peritubular capillary network in adult mice. Their conditional transgenic approach that overexpresses native Ang-1 specifically in mature renal tubules is an advantage over previous studies, in which conflicting results were attributed to different engineered forms of Ang-1 being used.
In collaboration with Drs. Scott Manson and Paul Austin, Division of Urology, Department of Surgery, the study demonstrated for the first time that conditionally enhanced tubular expression of Ang-1 halts the progression of renal fibrosis associated with reduced renal inflammation and stimulates the growth of peritubular capillaries in the obstructed kidney of a murine unilateral ureteral obstruction (UUO) fibrosis model. They also showed that overexpression of the vascular growth factor postnatally does not cause any structural abnormalities in the tubules or capillaries.
“Microvascular rarefaction is a central component of renal fibrosis and chronic kidney disease,” says Dr. Chen. “We feel excited that angiopoietin-1 could be a potential therapeutic agent for targeting microvasculature injury in renal fibrosis without compromising the physiologically normal vasculature in humans.”
Read the full article here.