Glia1 Cell Stem Cell
An exciting, new study by researchers at Washington University School of Medicine in St. Louis, MO, Brigham and Women’s Hospital, Harvard Medical School in Boston, MA, and RWTH Aachen University in Aachen, Germany, has identified the cells responsible for the vascular calcification seen in CKD. This finding is of particular importance because vascular calcification is a significant contributor to cardiovascular disease, which is the leading cause of death for patients with CKD.
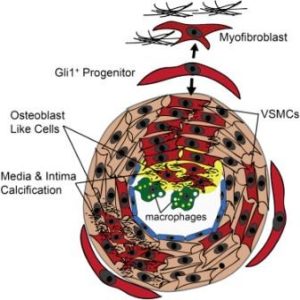
The study by Kramann et al., published September 8, 2016, in Cell Stem Cell, found that Gli1+ adventitial mesenchymal stem cell (MSC)-like cells are what drive CKD-induced arterial calcification. The study also showed that genetic ablation of the Gli1+ stem cells before the onset of CKD dramatically reduced vascular calcification.
Gli1+ stem cells reside in the adventitia, the outer layer of the artery. They are adult stem cells and have the potential to transform to different types of cells, including smooth muscle, fat and bone cells. The Gli1+ cells normally function to heal damaged blood vessels by becoming new smooth muscle cells. However, under the conditions of CKD, Gli1+ cells can differentiate into osteoblast-like cells that drive the process of calcification.
Senior author Benjamin D. Humphreys, MD, PhD, Associate Professor and Chief, Division of Nephrology, in an interview with Julia Evangelou Strait from Washington University’s The Source, said: “Now that we have identified Gli1 cells as responsible for depositing calcium in the arteries, we can begin testing ways to block this process. A drug that works against these cells could be a new therapeutic way to treat vascular calcification, a major killer of patients with kidney disease. But we have to be careful because we believe these cells also play a role in healing injured smooth muscle in blood vessels, which we don’t want to interfere with.”
See full article and interview.