Associate Professor Sanjay Jain, MD, PhD and Assistant Professor Moe Mahjoub, PhD, in the Division of Nephrology will each receive a micro-grant from the Children’s Discovery Institute (CDI) Zebrafish Models for Pediatric Research Services Cooperative (ZRSC).
The CDI, a partnership between St. Louis Children’s Hospital and Washington University School of Medicine, was established in 2006 with a goal of changing the way pediatric research is conducted. The CDI funds research that targets some of the most devastating childhood diseases and disorders. Researchers are encouraged to “ask bold questions and take bold risks to uncover answers.”
The CDI established the ZRSC to fund new projects utilizing zebrafish to study pediatric disease and birth defects. The funds pay for services performed by the ZRSC core facilities (see below).
Dr. Mahjoub’s winning proposal, “Analysis of Ciliopathy Genes in Zebrafish,” aims to identify novel genes involved in the pathogenesis of human ciliopathy syndromes.
 The zebrafish has emerged as a powerful vertebrate model in which to query defects associated with ciliary dysfunction. As the embryos and larvae contain multiple different ciliated cell types, zebrafish has been used to study both sensory and motile cilia functions. Indeed, a number of zebrafish models of human ciliopathies have been established, and forward/reverse genetic approaches developed to interrogate the functional consequences of introduced mutations, in essence “humanizing” zebrafish for use as a model for human disease gene function and variability.
The zebrafish has emerged as a powerful vertebrate model in which to query defects associated with ciliary dysfunction. As the embryos and larvae contain multiple different ciliated cell types, zebrafish has been used to study both sensory and motile cilia functions. Indeed, a number of zebrafish models of human ciliopathies have been established, and forward/reverse genetic approaches developed to interrogate the functional consequences of introduced mutations, in essence “humanizing” zebrafish for use as a model for human disease gene function and variability.
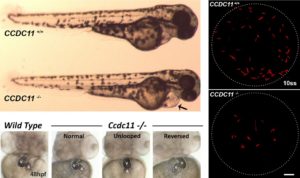
As an example of such an approach, the Mahjoub Lab recently characterized the role of a ciliopathy gene that was mutated in patients with respiratory disease and organ laterality defects. Using gene-targeting methods in zebrafish, they generated a number of independent null strains in this gene (CCDC11). Remarkably, these fish had similar phenotypic defects as those observed in the patients, including defective left-right asymmetry and reversed heart looping. They were thus able to study the ciliary defects associated with loss of this gene in motile ciliated cells of the Kupffer’s vesicle (embryonic node) and the multiciliated cells of the pronephric ducts during zebrafish development.
“These experiments highlight the power of zebrafish as a model of human ciliopathies, allowing us to rapidly move back and forth between disease gene identification and analysis of the consequences of these mutations in a developing vertebrate organism,” says Dr. Mahjoub.
Dr. Jain’s winning proposal will study causative variants leading to congenital anomalies of the kidney or lower urinary tract (CAKUT). Occurring in 1 in 250 live births, CAKUT are the most common cause of renal failure in children. In some cases, these are incompatible with life. Even after surgical correction, many patients progress to renal failure or have associated comorbidities that include skeletal, cardiovascular and hematopoietic systems.
“Despite the severe consequences of renal malformations in children, large gaps exist in our knowledge in delineating causative variants that lead to CAKUT in humans and how they affect long-term outcomes,” says Dr. Jain. “A few groups in the world, including us, have begun to survey genome wide alterations in children with CAKUT to better understand the mechanisms and etiology to help with management and treatment.”
However, one major obstacle delineates the functional relevance of mutations or variants discovered in patients. “The uncertainty in assessing causality of identified genetic changes limits proper care, therapy and counseling of patients,” says Dr. Jain. “We have completed and analyzed whole exome sequencing data of 120 patients with CAKUT that are from the local Midwest region (a unique US-based cohort) using state-of-the-art informatics tools. This award will be used to examine the functional significance of pathogenic variants we have identified.”
Dr. Jain points out that the ZRSC services are critical to efficiently introduce pathogenic variants using CRISPR/Cas9 mediated genome editing of target genes in zebrafish and determine causation and the underlying mechanisms. “If causality is confirmed in model systems, it immediately opens avenues for risk assessment, comorbidities that may be related to identified genetic mutations, genetic counseling and therapeutic options including small molecule targets to improve kidney function or prevent them from injury.”
The ZRSC core facilities include the Fish Facility (FF), Genome Technology Access Center (GTAC), Genome Engineering and iPSC Core (GEiC), WU High Throughput Screening Core (HTS), Molecular Vectors Core (MVC), WU Center for Cellular Imaging (WUCCI), and the Protein & Nucleic Acid Chemistry Laboratory (PNACL).