ADTKD is a genetic form of renal fibrosis, leading to chronic kidney disease (CKD). It represents as many as 25% of patients with inherited kidney disease, after exclusion of polycystic kidney disease and Alport syndrome. However, due to lack of a distinctive clinical feature and absence of diagnostic tests other than genetic testing, the prevalence of ADTKD is significantly underestimated.
ADTKD is caused by mutations in UMOD, MUC1, REN and HNF1B. UMOD encoding uromodulin is the leading mutated gene to cause ADTKD, and more than 135 UMOD mutations have been identified. ADTKD-UMOD is characterized by hyperuricemia, gout, alterations in urinary concentration, and progressive loss of kidney function. Currently there is no treatment.
The goal of ADTKD Clinic is to closely work with patients, patient organizations, pharmaceutical companies and international ADTKD consortiums to find the optimal treatment for ADTKD.
The ADTKD clinic is headed by Ying (Maggie) Chen, MD., Ph.D. Dr. Chen is the principal investigator of multiple grants including NIH-funded R01 and Department of Defense (DOD) grants, to advance treatment for genetic kidney disease. She is a standing member in American Society of Nephrology (ASN) and VA Merit Review study sections, as well as an elected member of the American Society for Clinical Investigation.
To find the cure for ADTKD, Dr. Chen’s lab has generated the mouse, human kidney cell and human induced pluripotent stem cell (hiPSC) models to recapitulate human ADTKD. Most importantly, her group has discovered a biotherapeutic protein MANF to treat ADTKD in mice.
To achieve the mission of our ADTKD clinic, we will offer personalized clinical management, as well as leverage state-of-the-art molecular phenotyping technology to identify novel drug targets and validate biomarkers.
Precision Management of ADTKD
- Offer free genetic sequencing to identify deleterious gene mutations.
- Establish a full range of molecular and clinical profiles for each patient, including DNA sequence, gene expression and histology from kidney biopsies, blood and urine samples, and human induced pluripotent stem cell (hiPSC)-derived kidney organoids.
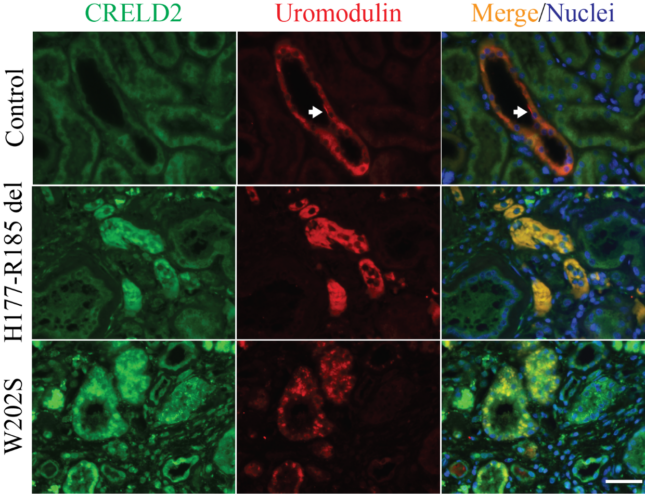
- Discover and validate blood and urine biomarkers, such as BiP and CRELD2 identified by us to monitor endoplasmic reticulum (ER) stress in ADTKD kidneys, in translational studies.
- Develop pioneering, highly mechanistic therapies. We will conduct high-throughput drug screening by collaborating with the Division of Pre-Clinical Innovation at NIH/NCATS, develop RNA-based therapy by working together with company, and establish CRISPR and adeno-associated virus (AAV)-based gene therapy.
- Enroll patients to future targeted clinical trials based on identified novel drug targets.

If you have ADTKD or unknown etiology of CKD with family history, please call 314-362-9096 for an appointment.