
Anuja Java, MD, assistant professor of medicine, Division of Nephrology, has been researching rare complement diseases and their involvement in kidney damage for nearly a decade. Little did she know when 2020 began that her expertise would put her in prime position to help tackle one of our greatest medical challenges – COVID-19.
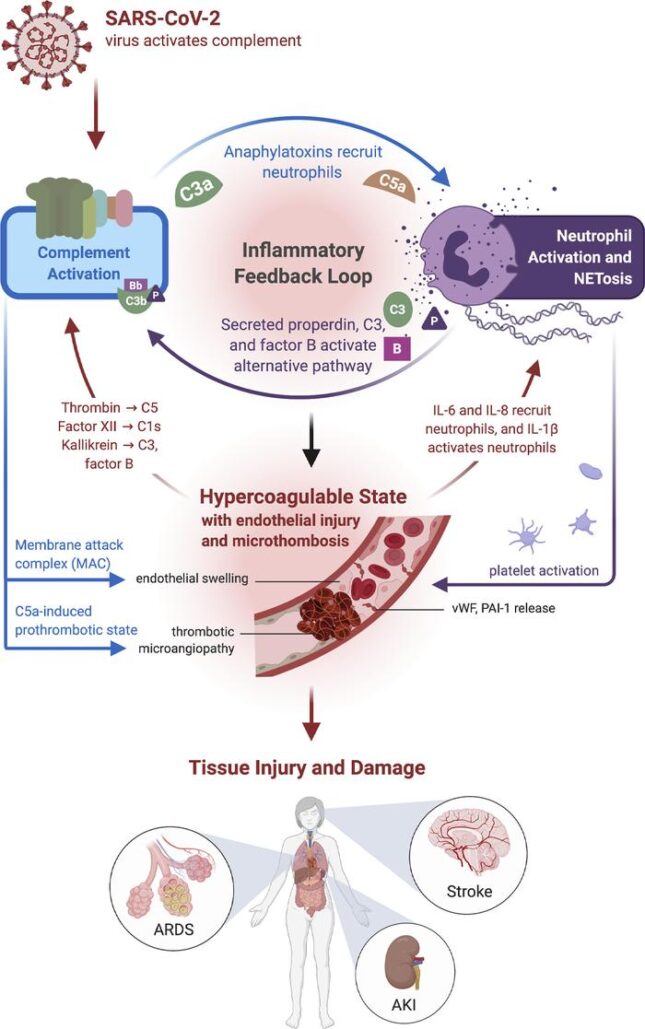
Early in the COVID-19 pandemic, it was suspected that complement activation played a critical role in the parthenogenesis of the disease. The complement system enhances the ability of antibodies and phagocytic cells to eliminate infectious microorganisms and is the first response of the host immune system to SARS-CoV-2, the acute respiratory syndrome coronavirus that causes the disease. In some patients, the complement system subsequently becomes overactive and results in, among other morbidities, severe inflammation and hypercoagubility, resulting in multiple organ failure and even death. Acute kidney injury is a well-known complication of COVID-19, and many patients end up requiring dialysis.
A recent review in JCI Insight by Dr. Java summarizes interactions between coronaviruses and the complement system. The review discusses SARS-CoV-2 and describes clinical trials that are underway for the treatment of severe COVID-19. “This is a very comprehensive review on this topic and has been very well received by the medical community,” says Java.
Java is a co-investigator on one of the clinical trials detailed in the review. The Phase 3 trial is examining the efficacy and safety of the drug ravulizumab in acute lung injury and cytokine storm associated with COVID-19 (ClinicalTrials.gov Identifier: NCT04369469). Ravulizumab is a recombinant humanized anti-C5 monoclonal antibody and is a terminal complement inhibitor. It is currently FDA-approved to treat two rare genetic diseases, atypical hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria (PNH), both of which cause life-threatening blood clots in small blood vessels.
“Ravulizumab is the longer acting version of eculizumab, a drug also FDA-approved for only a few diseases, one of which is aHUS,” says Java, who has extensive experience in the treatment and research of aHUS. “This disease, the focus of my research, is a ‘complementopathy’ that leads to acute kidney injury. Many features of aHUS overlap with COVID19.”
The ravulizumab trial involves investigators from 50 worldwide sites who plan to enroll 270 adult patients into the study. The goal is for about 20 patients to be enrolled at Barnes-Jewish Hospital. The study is sponsored by Alexion Pharmaceuticals, which manufactures ravulizumab under the brand name ULTOMIRIS®.
”The clinical trial is a direct result of our understanding of the role of complement system in the pathogenesis of COVID19 and how modulating this system by using complement inhibitors at the right time can potentially improve outcomes in these patients,” says Java.
Java began investigating the role of complement receptors and regulators in the pathogenesis of immune and inflammatory processes in the kidney when she was a WashU fellow in transplant nephrology, and she collaborates with former chief of WashU Rheumatology John Atkinson, MD, the Samuel B. Grant Professor of Clinical Medicine, under whom she trained. Atkinson, according to Java, is a Complement Guru! “I feel extremely fortunate to have trained under him as my mentor and thoroughly enjoy working with him now as a collaborator,” she says.
In a recent study with collaborators from WashU, the University of Massachusetts School of Medicine, Newcastle University, and Allergan, Java et al. detail for the first time a comprehensive functional assay of rare genetic variants in complement factor I (CFI) using a serum-based assay in advanced age-related macular degeneration. Identifying patients in the clinic with rare CFI genetic variants will help target those most likely to benefit from complement pathway-related therapies, thus providing a personalized medicine approach to treatment. The article is published in the journal Translational Vision Science & Technology (tvst, August 2020, Authors: Anuja Java, Peter Baciu, Rafael Widjajahakim, Yun Ju Sung, Jae Yang, David Kavanagh, John Atkinson, and Johanna Seddon). This work was also presented at the American Society of Human Genetics (ASHG) conference this year.
In addition to her work as a research scientist, Java is also director of the new Kidney Transplant Clinic at the Veterans Affairs (VA) St. Louis Health Care System. The clinic is for patients who have received a kidney transplant and for those with chronic kidney disease who need evaluation to determine their candidacy for a transplant. The service is a valuable and convenient resource for local veterans.

If you registered for the 2020 ASN Kidney Week Reimagined meeting, you can access the session on Thrombotic Microangiopathy in 2020: Complement at the Forefront, which Java co-moderated.
Read the JCI Insight review here. Authors: Java A, Apicelli AJ, Liszewski MK, Coler-Reilly A, Atkinson JP, Kim AHJ, Kulkarni HS (Kim and Kulkarni are co-senior authors).
You can find physician-scientist Anuja Java @anuja_java and @WUNephrology on Twitter. Please visit and “Like” our Facebook page.