The spectrum of congenital anomalies of the kidneys and urinary tract, known as CAKUT, represents more than 20% of all birth defects and constitutes the largest cause of kidney failure in children.
Masato Hoshi, PhD
A new study by Dr. Masato Hoshi, a Senior Scientist in the laboratory of Associate Professor of Medicine, Sanjay Jain, Division of Nephrology, provides novel insights that may explain the mechanisms of many CAKUT. The article, Reciprocal Spatiotemporally Controlled Apoptosis Regulates Wolffian Duct Cloaca Fusion, was published as a Brief Communication in the March 2018 issue of JASN.
The Wolffian duct (WD) is an embryogenic structure critical to the formation of the kidney and its connection to the primitive bladder, the cloaca. During development, insertion of the WD into the cloaca and formation of the ureteric bud from progenitor cells in the WD are two key events for establishing normal plumbing of the urinary system and formation of the mammalian kidney. The ureteric bud goes on to initiate kidney development.
Defects in the processes that establish this connection between the upper and lower urinary tract result in a wide range of CAKUT.

Sanjay Jain, MD, PhD
RET receptor tyrosine kinase activation results in the phosphorylation of key tyrosines (Y1015 and Y1062) that bind specific intracellular adaptors and cause activation of signaling cascades, that regulate cell survival and apoptosis. Dr. Jain has previously shown that disruption of RET signaling can cause several types of CAKUT in mice and humans.
Here, the research team used innovative models of RET mutant mice, three-dimensional confocal immunofluorescence, and three-dimensional X-ray nano-computed tomography imaging in conjunction with whole urinary tract cultures to study specific RET-mediated pathways and cellular mechanism that regulate early WD tip fusion with the cloaca.
They discovered that a novel process of spatiotemporally regulated apoptosis in both the WD and cloaca was necessary for proper WD-cloacal fusion. In this model, when the WD reaches the cloaca, WD cells at the point of contact with the cloaca apoptose, followed by induction of cloacal apoptosis. Apoptosis of both structures at the contact site was necessary for their fusion.
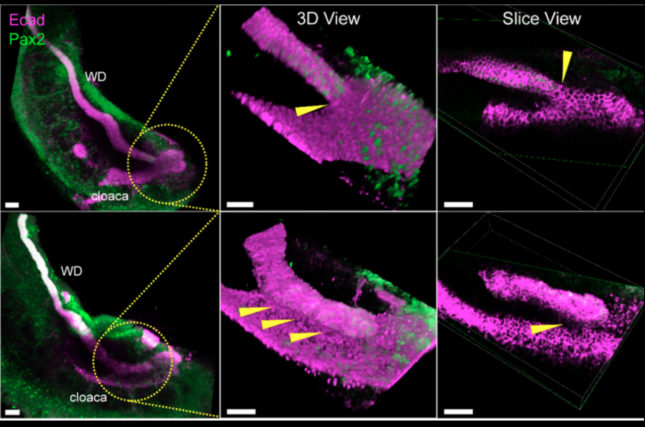 Abrogation of RetY1062F-activated MAPK signaling prevented the WD from reaching the cloaca due to accelerated apoptosis in the WD tip. Cloacal apoptosis did not occur in these mutants due to loss of inductive signals from the failed contact with the dying WD tip.
Abrogation of RetY1062F-activated MAPK signaling prevented the WD from reaching the cloaca due to accelerated apoptosis in the WD tip. Cloacal apoptosis did not occur in these mutants due to loss of inductive signals from the failed contact with the dying WD tip.
In RetY1015F mutants, MAPK signaling is activated, preventing natural apoptosis at the WD tip at the site of contact with the cloaca. Even in these mutants, cloacal apoptosis did not occur as naturally programmed cell death at the site of contact in the WD was absent.
Both mutant animal models displayed a spectrum of ureter and kidney anomalies reminiscent of CAKUT. In vitro studies supported the finding that aberrant RET signaling affects elongation of the WD tip and its fusion with the cloaca through interdependent apoptosis.
These findings may help explain the underlying pathogenetic mechanisms responsible for conditions such as agenesis, hypoplasia and ureter defects in CAKUT patients.
Authors: Masato Hoshi, Antoine Reginensi, Matthew S. Joens, James A. J. Fitzpatrick, Helen McNeill, and Sanjay Jain.