Renal fibrosis, a hallmark of chronic kidney disease (CKD), is characterized by the accumulation of excess extracellular matrix proteins secreted by myofibroblasts. Unfortunately, there are no anti-fibrotic drugs approved for treatment of CKD.
A new study headed by researchers in the Humphreys Laboratory in the Division of Nephrology at Washington University, however, is laying the groundwork for the use of adeno-associated virus (AAV) vectors to inhibit renal fibrosis via targeted gene therapy. Their “targets” are the mesenchymal progenitors in the kidney interstitium that differentiate into myofibroblasts.
 The adeno-associated virus (AAV) vector has become a powerful tool in delivering genes to mammalian cells because of its ability to infect both dividing and non-dividing cells, its low immune response and a favorable safety profile in humans. The ability to deliver genetic material specifically to myofibroblast progenitors could allow new therapeutic approaches to treat kidney fibrosis.
The adeno-associated virus (AAV) vector has become a powerful tool in delivering genes to mammalian cells because of its ability to infect both dividing and non-dividing cells, its low immune response and a favorable safety profile in humans. The ability to deliver genetic material specifically to myofibroblast progenitors could allow new therapeutic approaches to treat kidney fibrosis.
The researchers evaluated a panel of six AAV subtypes, both natural and synthetic. They identified the synthetic AAV Anc80 as a promising candidate to target the kidney mesenchymal cell lineage. Anc80, which had been engineered to reduce antigenic epitopes, allowed efficient gene transfer to kidney pericytes, mesangial cells, and juxtaglomerular apparatus without toxicity. Their results determined that Anc80 transduced kidney myofibroblast progenitors that undergo proliferative expansion during fibrotic injury.
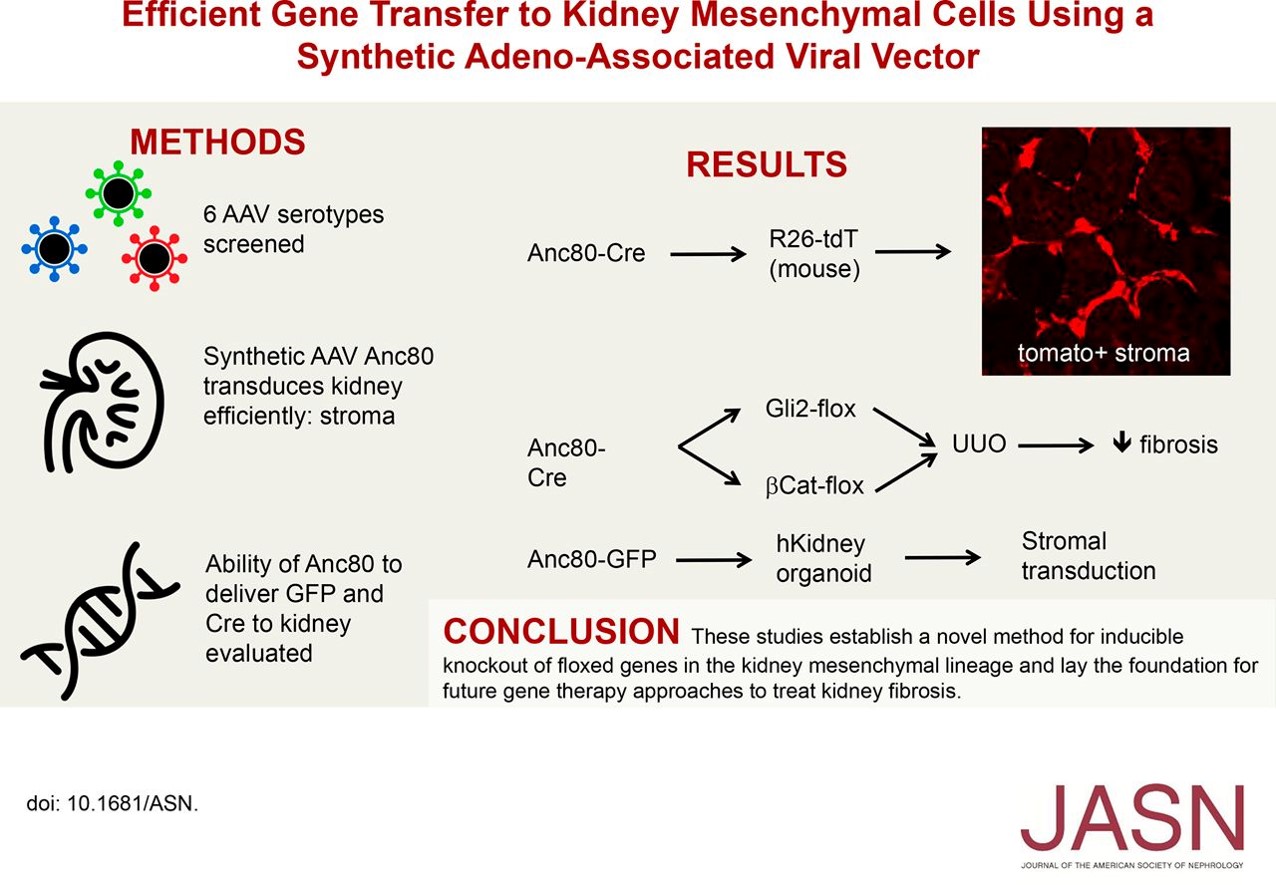
 The group had previously shown that the Gli2 protein is required for myofibroblast proliferation during kidney fibrosis by breeding Gli2 flox mice with inducible Cre driver mice. In the current study, they confirmed that pericyte-specific knockout of Gli2 by AAV-Cre ameliorated kidney fibrosis without using Cre driver mice.
The group had previously shown that the Gli2 protein is required for myofibroblast proliferation during kidney fibrosis by breeding Gli2 flox mice with inducible Cre driver mice. In the current study, they confirmed that pericyte-specific knockout of Gli2 by AAV-Cre ameliorated kidney fibrosis without using Cre driver mice.
To determine if the canonical Wnt–β-catenin signaling is implicated in myofibroblast proliferation and differentiation during kidney fibrogenesis, they used Anc80-Cre to knockdown β-catenin. Pericyte-specific gene targeting of β-catenin, indeed, ameliorated kidney fibrosis after unilateral ureteral obstruction injury to the kidney.
“This is the first report to show the pericyte-specific contribution of Wnt–β-catenin signaling to kidney fibrosis,” says first author Yoichiro Ikeda, MD, PhD. Dr. Ikeda was a postdoctoral research fellow in the Humphreys laboratory at the time of the study. He is currently Assistant Professor at the University of Tokyo, Graduate School of Medicine (ikeda-tky@umin.ac.jp).
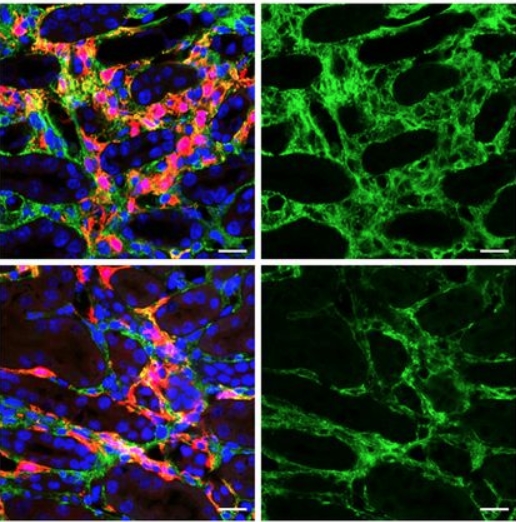 Lastly, the group determined that Anc80 was capable of transducing not only murine mesenchymal cells, but mesenchymal cells in human kidney organoids, as well.
Lastly, the group determined that Anc80 was capable of transducing not only murine mesenchymal cells, but mesenchymal cells in human kidney organoids, as well.
While Anc80 is identified as a promising candidate for gene therapy applications targeting the kidney mesenchymal cell lineage, the researchers point out that several obstacles must be overcome before using it in a clinical setting. Continued engineering of AAV tropism (i.e. the ability of a given virus to productively infect a particular cell) is needed to achieve kidney specificity. In addition, because of the slow progressive nature of CKD, repeated injections of the vector may be needed, raising the possibility of immunogenicity; because Anc80 is a synthetic virus, repeated injections could lead to acquired immunity.
The study, Efficient Gene Transfer to Kidney Mesenchymal Cells Using a Synthetic Adeno-Associated Viral Vector, is published in the September 2018 issue of JASN (online ahead of print in July). The publication has been featured in 25 news outlet articles (including Science Daily and NewsWise) and has been commented upon by 28 tweeters. In addition, the story ranks #21- with regard to an Attention Score – out of 3,296 JASN manuscripts tracked by Altmetic.
Manuscript authors: Yoichiro Ikeda, Zhao Sun, Xiao Ru, Luk H. Vandenberghe and Benjamin D. Humphreys.