
Dr. Benjamin Humphreys
The results of an international collaboration of researchers including Dr. Benjamin Humphreys, Division of Nephrology at Washington University, and Dr. Rafael Kramann, Division of Nephrology and Clinical Immunology at RWTH Aachen University in Germany, published in the May issue of JCI insight, settles the years-old controversy of the origin of scar-forming macrophages in the kidney.
A hallmark of chronic kidney disease, renal fibrosis – or scarring – is characterized by the accumulation of excess extracellular matrix proteins. While myofibroblasts are known to be the cells that secrete the scar matrix, their origin has been the subject of intense discussion. In particular, there is a question as to whether circulating hematopoietic or mesenchymal cells derived from bone marrow contribute to the pool of resident renal myofibroblasts.
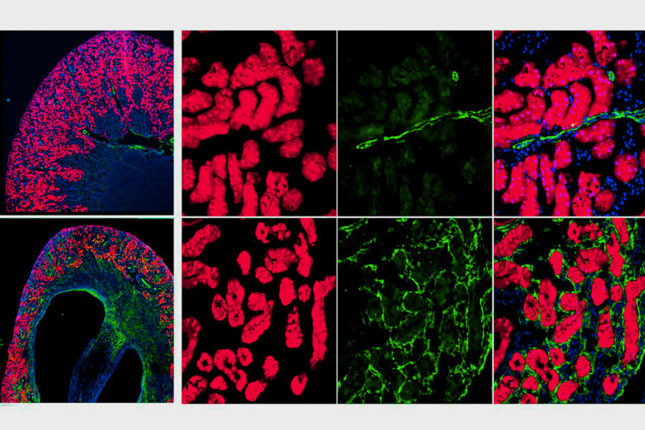
The group used two approaches to determine the source of the renal myofibroblasts. A parabiosis mouse model allowed them to fate-trace all the cells from one mouse parabiot and induce kidney fibrosis in the other. They also used single-cell RNA sequencing to confirm genetic fate-tracing parabiosis results.

Dr. Raphael Kramann
“Our work indicates that there are indeed some circulating cells that acquire expression of the myofibroblast marker ACTA2 in fibrotic kidneys,” says first author Dr. Kramann, Professor of Medicine and Chair of Nephro-Cardiology, RWTH Aachen University. “Single cell RNA-sequencing indicates that these cells are monocyte derived.”
An even more important finding, however, was that these cells actually do not secrete much extracellular matrix. “They secrete many inflammatory cytokines, suggesting that they do not directly contribute to the myofibroblast pool but rather activate resident mesenchymal cells via proinflammatory stimuli.” Thus, a paracrine communication between circulating monocytes and resident renal myofibroblasts may drive fibrosis.
Single-cell RNA sequencing also confirmed that the majority of kidney myofibroblasts responsible for fibrosis are of resident kidney origin.
“Understanding the cellular origin of these cells will guide the development of novel targeted therapeutics that can inhibit these cells and then potentially ameliorate progression of chronic kidney disease,” says Kramann.
Read the full article, Parabiosis and single-cell RNA sequencing reveal a limited contribution of monocytes to myofibroblasts in kidney fibrosis, here. Authors: Rafael Kramann, Flavia Machado, Haojia Wu, Tetsuro Kusaba, Konrad Hoeft, Rebekka K. Schneider, and Benjamin D. Humphreys.